Over the past several months, the Good Science Project has been commissioning a series of policy briefs on R&D reform. The Federation of American Scientists graciously agreed to partner with us on the publication of these policy briefs, which are available here.

There are many great ideas here, some of which could make an enormous difference to our health and wellbeing.
A quick rundown:
Reform Clinical Trials
Policy briefs aren’t known for being emotional and heart-wrenching. This one is an exception: Jake Seliger and his wife (now widow) Bess Stillman collaborated to write about the FDA and clinical trials, based on their experience dealing with Jake’s cancer. Jake passed away after writing the first draft, while Bess was pregnant with their first and only child.
I had followed Jake’s excellent blog for many years, and had seen posts about his ongoing struggle with cancer. It was quite lucky and fortuitous that I had the chance to meet him and Bess last summer at a conference, and I found it moving that they found the time and energy, even at death’s door, to write up ideas on how to improve the medical system for other people in the future. As they say, “he was good people.”
They have multiple recommendations: 1) the FDA should to more to conditionally approve early-phase treatments for terminal cancer patients, 2) the FDA and NIH should work to radically improve ClinicalTrials.gov with better accessibility and data so that terminal patients can find the right trials, and 3) the FDA should do more to mandate remote access and telemedicine so that patients across the country can much more easily enroll in clinical trials without incredibly burdensome travel.
Among many pointed observations:
- “It’s easier to find a dress to my exact specifications out of thousands on H&M,com than it is to find a clinical trial”
- “Finding a clinical trial already feels a bit like online dating, except if you get the wrong match, you die.”
A New Bell Labs
Jeff Tsao of Sandia National Laboratories (and a co-author of an excellent book on metascience) wrote about reinvigorating the Bell Labs model in today’s economy.
In a nutshell, his idea is that government and philanthropy should partner with large corporations to co-sponsor research labs. The corporate affiliation would (like AT&T) provide an applied setting giving rise to many cutting-edte research problem. At the same time, the government/philanthropic sponsorship would allow researchers to have the intellectual freedom to make new basic discoveries and pivot towards curiosity-driven opportunities, without being constrained by the corporation’s bottom line.
The National Academies is hosting a small meeting on this idea soon, and top corporations and government policymakers are participating. Tsao’s policy brief is here.
Bounty Hunters for Science
The idea here is straightforward: Scientific fraud is too common, and no one tries to expose it, except for a few intrepid folks who are willing to withstand immense criticism and the constant threat of lawsuits.
Under the federal False Claims Act, it’s possible for whistleblowers to be rewarded after a lengthy and difficult lawsuit, but we need to make it much more immediately rewarding for people to report on potential scientific fraud or other malpractice. Chris Said (a data scientist in industry) has a policy brief on that idea here.
Report All Null Results
A significant problem in science is that researchers (often responding to the incentives of journals and funders) don’t write up and publish null/negative results, instead choosing to move on to the next thing. But that means that the rest of the field doesn’t learn from those results, and moreover there’s an incentive to commit fraud or engage in p-hacking in order to come up with positive results.
Randy Ellis (a post-doc at Harvard Medical School) has a policy brief recommending that federal policymakers should do more to mandate publication of null results. This is a no-brainer.
Sponsor Better Nutrition Research
Way too much nutrition research is of fairly low-quality. And perversely, the federal government has been scaling back on the best nutrition research.
Kevin Klatt (an assistant research scientist at Berkeley) has a bunch of recommendations for how Congress, NIH, and USDA could respond to these challenges, most importantly including the creation of Centers of Excellence in Human Nutrition. Well worth reading if you care about “Making America Healthy Again.”
Improve Health Data
Most clinical guidelines aren’t actually supported by high-quality evidence. We need more and better clinical evidence, which means more trials and other studies, which in turn require more and better data.
Laura Esserman (one of the most renowned clinical trialists in the US) and her colleague Ali Abbasi wrote a policy brief on how agencies like CMS and NIH could radically improve healthcare data (e.g., electronic health records).
Improve FDA’s Use of Surrogate Endpoints
The FDA often approves drugs not based on whether they improve actual health or lifespan, but based on whether they improve so-called surrogate endpoints (such as blood pressure readings). But surrogate endpoints aren’t what patients really care about—after all, if you still have a heart attack or die, will you care that at least your blood pressure reading went down for a while?This is a real issue, because many of the surrogate endpoints that the FDA has used don’t have solid evidence behind them. Even LDL cholesterol has a mixed track record, with several examples of treatments that lowered LDL without improving actual cardiovascular outcomes (and in one case making them substantially worse):
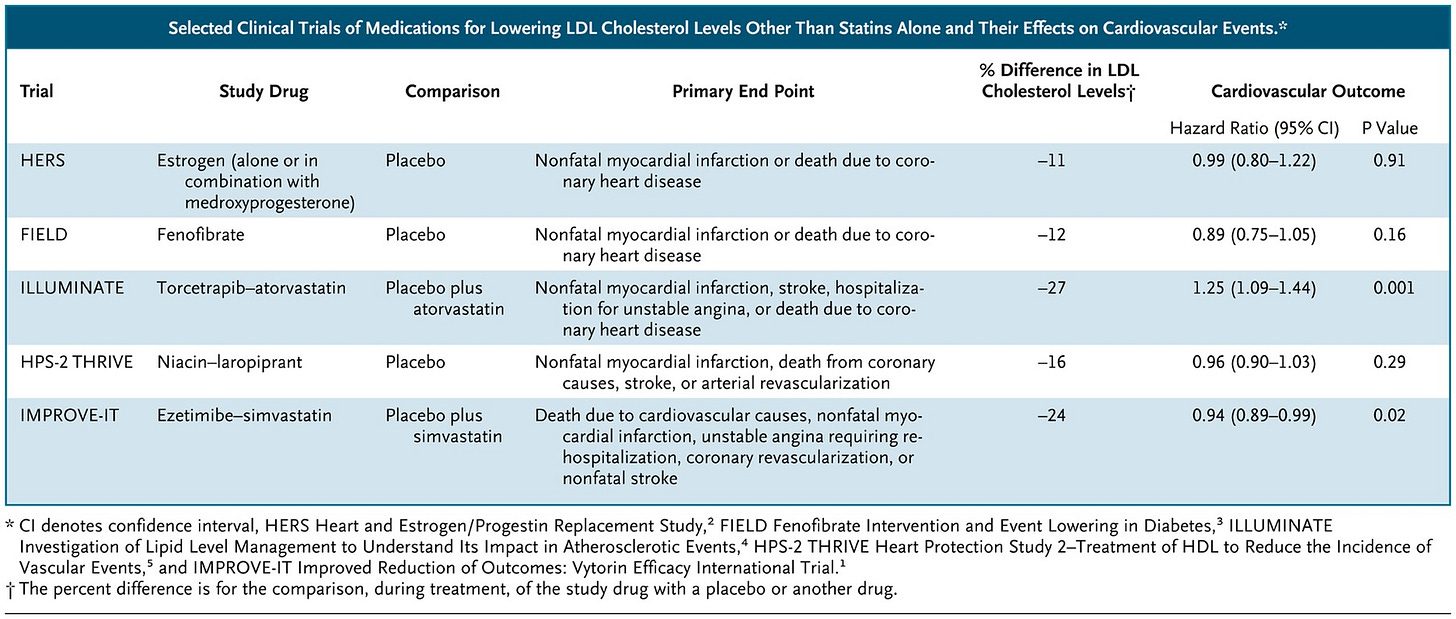
Some top scholars at Yale and Emory (Reshma Ramachandran, Josh Wallach, and Joe Ross) wrote a policy brief on this issue. They recommend that: 1) the FDA should be much more clear about the strength of evidence (or lack thereof) for surrogate endpoints, 2) multiple other agencies (e.g., NIH, PCORI, CMS) should sponsor and conduct much more research on whether and how well surrogate endpoints work, and 3) Congress should require more rigorous science on surrogate endpoints.
Measuring and Reducing Bureaucracy
DOGE has been sucking up a lot of bandwidth over the past several months, and despite its supposed mission of reducing bureaucracy, it ended up vastly increasing bureaucracy in many ways.
We need to do the opposite of DOGE—that is, we need a real Department of Government Efficiency that isn’t obsessed with waste/fraud/abuse, but instead focuses on actually improving government efficiency and reducing the bureaucratic burden on science.
Michele Zanini was formerly with RAND and McKinsey, and has co-written a bestselling book on reducing bureaucracy based on his experience with many corporations. Zanini proposes that the White House should initiate an effort to quantify the bureauractic burden across science agencies (e.g., NIH, NSF, NASA, DOD), and figure out exactly where the most pressing burdens are actually coming from. That is, a true cost-benefit analysis of bureaucracy would help us figure out which bureaucratic burdens to cut or eliminate.
His policy brief is here. It should hopefully lead to real reform that streamlines science.
Improve Federal Grantmaking to Science
Neil Thakur (a former NIH official) wrote up two related ideas with Richard Ikeda (also a long-time NIH official who passed away in late 2024).
Their first idea is that the White House OSTP should ask agencies to use AI as to all grant applications, to “predict the future of science, enhance peer review, and encourage better research investment decisions by both the public and the private sector.” This could improve how we allocate science funding (and decide which areas are over- versus under-supported), how applicants prepare their proposals, how external observers are able to analyze trends, and more.
Their second idea is that research grants should be given digital identification, such as ORCID and DOI. This would make it much easier to track the results from any given federal research grant. Also a no-brainer.