
One of the biggest problems in scientific funding today is that younger scientists are increasingly shut out of funding, and are forced to stay in “training” for years on end or else drop out of academia. I recently met a young biology professor at a major university who did not want to be quoted by name: He/she had recently gotten a first NIH grant at age 40, after spending 8 years doing postdocs. He/she said that of their friends and cohort members in their early 40s, most were divorced, had no children, and had spent their entire life to that point either in school or in underpaid jobs.
This doesn’t sound like an attractive path to many college students, let alone people who have other options such as tech, finance, consulting, or even medical school (which also has long hours and many years spent in training, but at least results in a high-paid career eventually).
Consider the following graphs from Mike Lauer’s blog post last year:
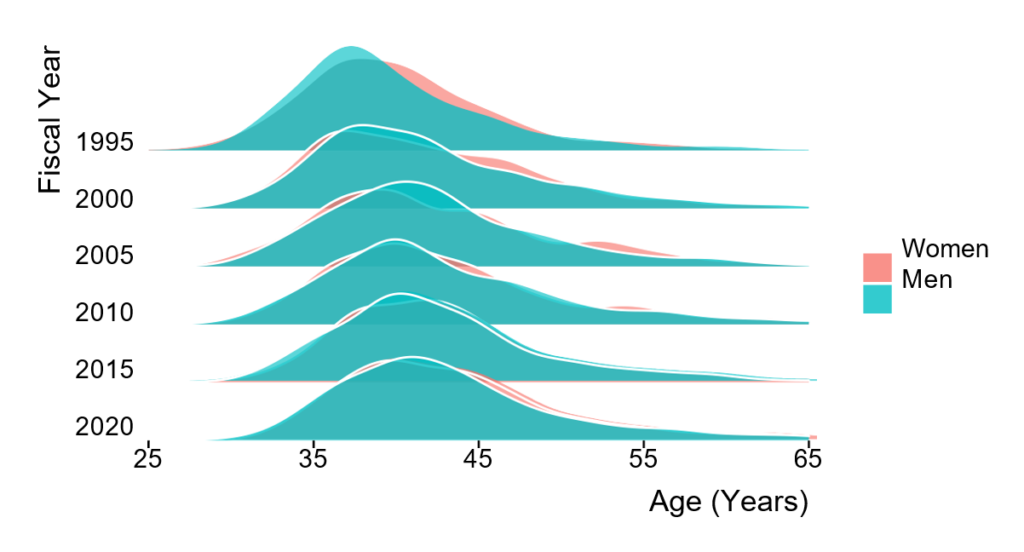
Here we see the age distribution of first-time NIH recipients of R01 grants. As recently as 1995, the distribution centered around people’s mid-30s. But in 2020, the median age for a first-time recipient was 42. Only a few lucky folks got grants before age 35.
Another graph, this one from a 2015 blog post (I was unable to find more up-to-date information):
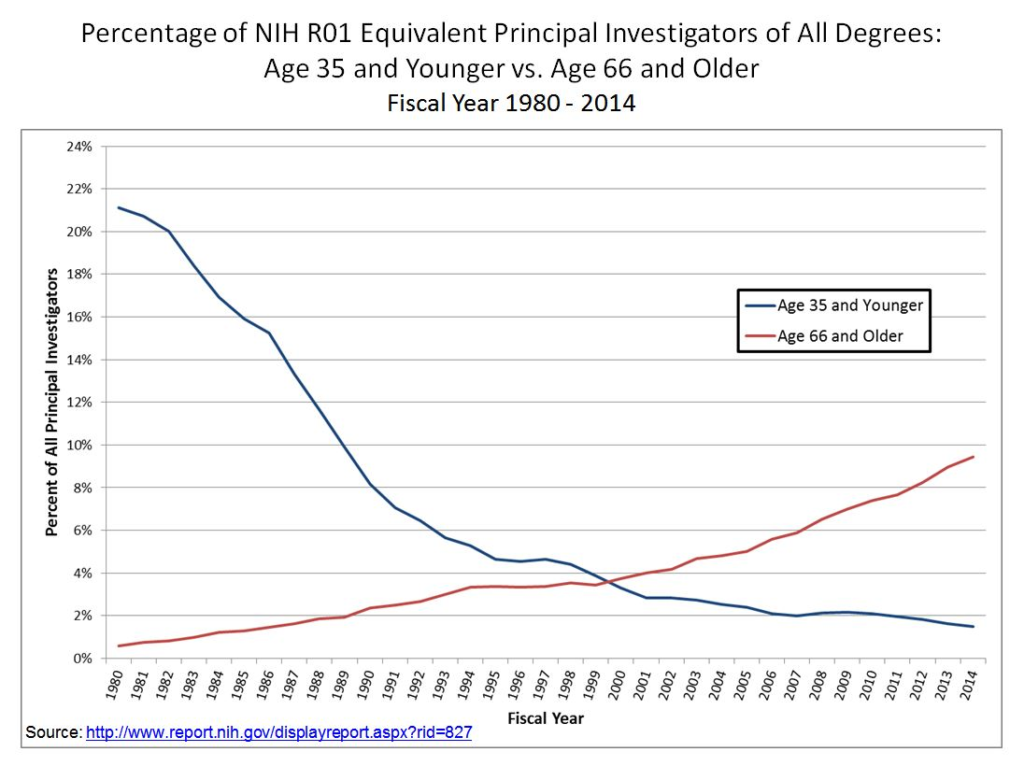
Here, we can see that the number of NIH grantees under 35 has plummeted 10-fold since 1980, while the number of grantees past retirement age has risen some 10-fold.
The NIH has been trying for a few years to rectify this disparity. In 2017, it launched a Next Generation Researchers Initiative with several grant categories meant to help early stage investigators. To be clear, that term (“early stage investigator”) is defined to include anyone who is up to 10 years out of their PhD or the “end of post-graduate clinical training, whichever is later.” (The latter bit is for MDs.)
If people often complete a biomedical PhD around age 28-30, that means you can still be an “early-stage” researcher until 38-40.
How many young folks (by this definition) does the NIH support? The latest numbers show that in 2020, the NIH gave first-time R01s (or equivalent) to 1,412 early stage investigators.
This may sound like a lot, and it is definitely more than in prior years. So let’s acknowledge that progress on NIH’s part.
But think about the number of biomedical doctorates produced in the US every year for the past 10+ years. Table 1-3 from NSF’s “Survey of Earned Doctorates” shows that in 2011, there were 8,152 doctorates; in 2016, there were 8,863; and in 2021, there were 8,149.
In other words, the US has been producing over 8,000 biomedical doctorates per year on a steady basis.
Most of those people wanted a career in academia at some point. If the NIH only awards 1,412 first-time R01s in a given year, while there were over 8,000 doctorates entering the workforce, that means that only about 17% of the graduates per year are finding NIH-funded employment.

Some folks might find academic positions with startup packages, but the rest either have to go into industry or else stick around for one or more post-docs for the next several years.
But within 5 years, there will be another 40,000+ PhDs flooding into the academic market to compete both for postdocs and academic positions!
At some point, the number of biomedical doctorates awarded in a given year has to match the number of academic positions (mostly funded by NIH grants) that come open. When there are way too many PhDs competing for the same NIH grants and academic positions, that doesn’t lead to a stable job market.
Nor does it lead to good research! As the famous “Rescuing Biomedical Research” article pointed out in 2014, this sort of hypercompetition for grants and jobs directly harms both reproducibility and innovation! It harms reproducibility by encouraging folks to cut corners in the desperate attempt to secure jobs and funding:
As competition for jobs and promotions increases, the inflated value given to publishing in a small number of so-called “high impact” journals has put pressure on authors to rush into print, cut corners, exaggerate their findings, and overstate the significance of their work. Such publication practices, abetted by the hypercompetitive grant system and job market, are changing the atmosphere in many laboratories in disturbing ways. The recent worrisome reports of substantial numbers of research publications whose results cannot be replicated are likely symptoms of today’s highly pressured environment for research
And hypercompetition harms innovation by encouraging folks to study safe, marginal topics:
Competition in pursuit of experimental objectives has always been a part of the scientific enterprise, and it can have positive effects. However, hypercompetition for the resources and positions that are required to conduct science suppresses the creativity, cooperation, risk-taking, and original thinking required to make fundamental discoveries. . . . The system now favors those who can guarantee results rather than those with potentially path-breaking ideas that, by definition, cannot promise success. Young investigators are discouraged from departing too far from their postdoctoral work, when they should instead be posing new questions and inventing new approaches. Seasoned investigators are inclined to stick to their tried-and-true formulas for success rather than explore new fields.
Hypercompetition is killing two birds with one stone–in the sense that it’s making everything worse.
Not only that, the people who survive in a hypercompetitive system might be optimizing for the wrong things. While hard evidence is difficult to come by, I know a neuroscientist who left academia, and who told me that the best and the brightest in neuroscience often do the same. Why? Precisely because they are talented and ambitious, they spend their graduate or post-doc years trying to tackle the most difficult problems in the field–and as a result, don’t churn out a long string of publications that would help them get the first job/grant.
One of the most important things we could do to improve biomedical science is bring the number of trainees (graduate students and post-docs) and the amount of likely future funding into balance.
There are only two ways that could happen: 1) Cut the number of trainees, or 2) increase the NIH budget devoted to trainees while holding the number of trainees constant.
The latter is more palatable, although it would take many years. Since NIH funding is ultimately zero-sum, you can’t just announce that 20% of funding is going to early-career folks next year. That would require taking money away from senior folks, and it won’t happen any more than the Grant Support Index did.
But we can’t keep churning out 8,000+ biomedical PhDs every year with only 1,400 or so NIH grants on offer. That math doesn’t work unless 80% of PhDs don’t want to enter academia at all.
NIH needs to seriously consider what to do about this. Bandaids aren’t going to fix this kind of mismatch.
***
A final note: This essay is meant to set up the problem. The solution? Well, there are a number of potential policies that we will explore in future essays. Stay tuned.